From March 2020, the packaging of our products will be improved to reduce storage space. In addition, the format of the label has been modified to adapt it to the new legal requirements of international regulation, to improve the information and the traceability of the product as well as the safety in its use.
Below are all the points you need to know about this change.
Why do we change the packaging and labelling?
Talladium España S.L. has started the transition process to meet the new regulatory requirements. During this phase, both the previous and the new labelling and packaging will comply with the requirements so that the products supplied during this phase can be marketed without problems.
What are the changes in the product packaging?
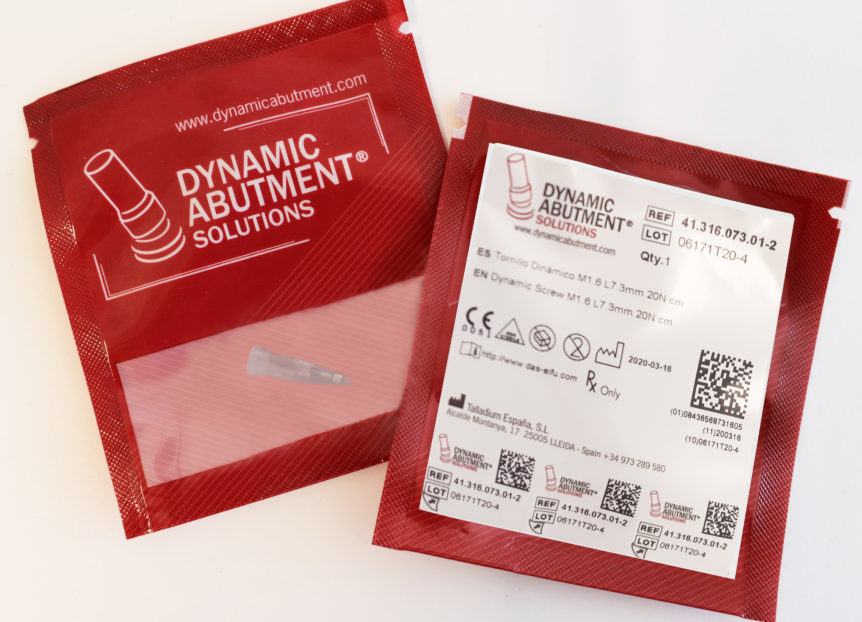
Until the moment the products of Talladium España S.L. were packed in a thermosealed blister. The new package is a lighter sachet that allows the storage of the product in a much smaller space.
What are the changes in labelling?
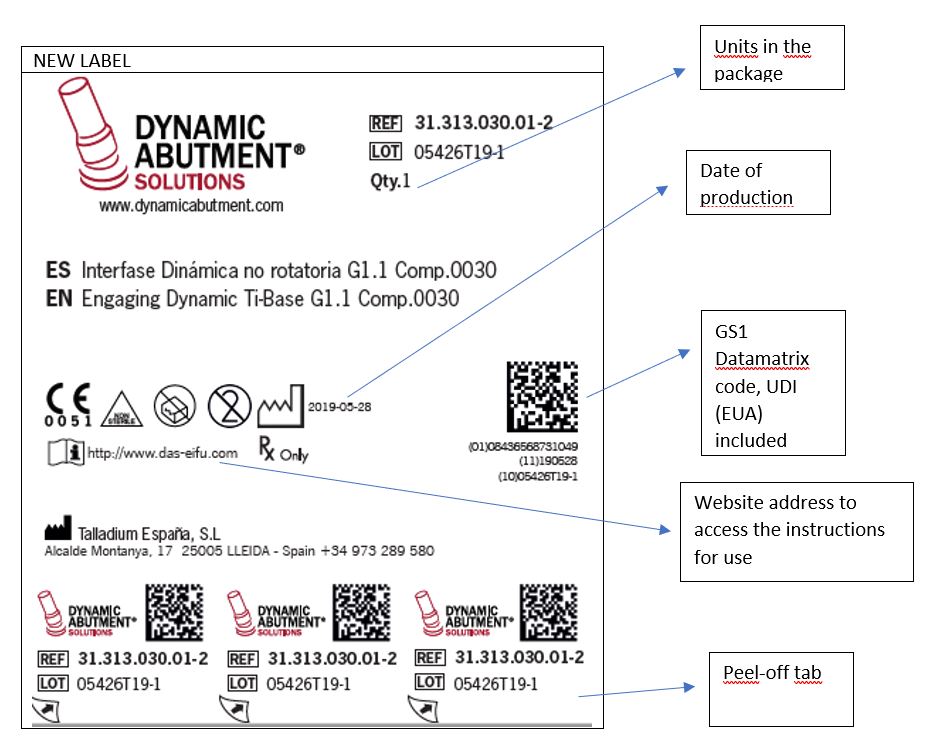
- Indication of the date of manufacture (Year-Month-Day / YYYY-MM-DD)
- “Material” is removed from the label. This information is included in the product descriptions and/or instructions for use.
- The units contained in the package are indicated (Qty.)
- The website address where the user can access and download the instructions for use of the product is added (www.das-eifu.com).
- The old QR code is replaced by a GS1 Datamatrix code. The content of the code has changed (explained in more detail at the end of this document).
- The product label has 3 tabs containing information about the traceability of the device. These tabs can be peeled off and included in the patient card as well as in the patient’s record.
How will these new formats be?

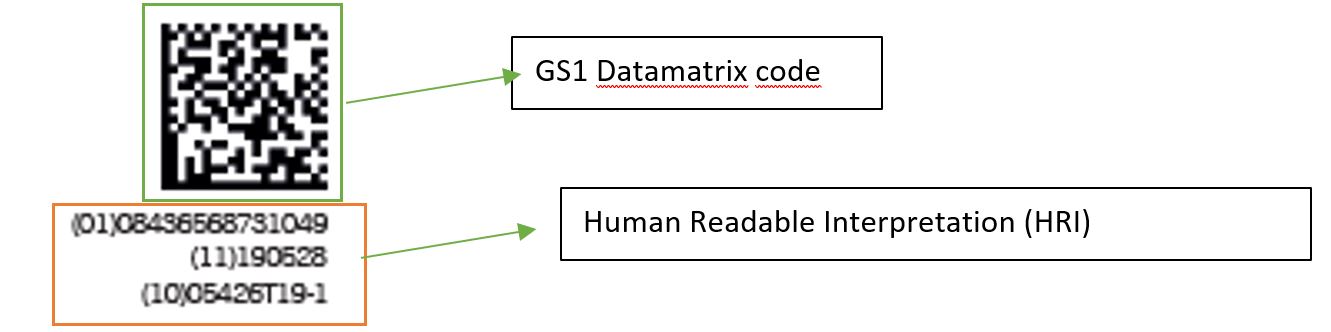
What is a GS1 Datamatrix code?
The GS1 DataMatrix is a 2-dimensional barcode. This type of code can contain a lot of information and is easily readable even when printed in small dimensions. It has a high reading reliability thanks to the 2-dimensional information system. The code consists of black and white cells that form a square or rectangular shape, each cell represents one piece of information.
Why a GS1 Datamatrix code?
The code is approved by ISO/IEC 16002. This standard ensures that all data matrix users (encoders and decoders) use the same code.
The GS1 standard, approved by the FDA and the European Commission, is used to generate this code in order to meet applicable legislation.
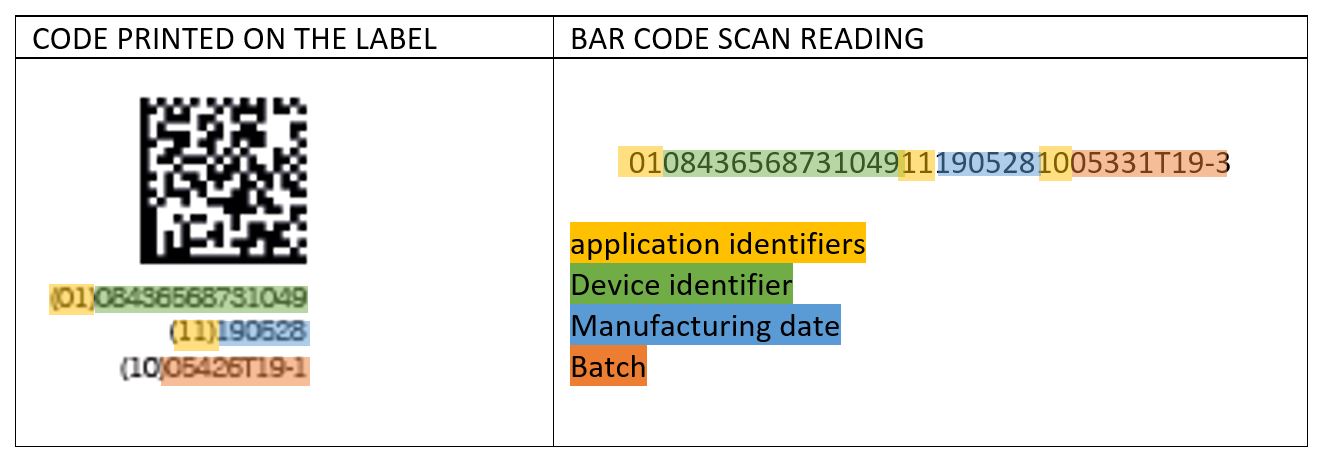
What information does the code contain?
The code consists mainly of two parts:
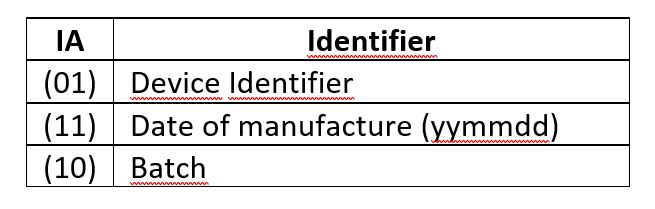
– UDI-DI (Device Identifier): This part of the code includes the manufacturer’s identification, the product reference and the units contained in the package.
– UDI-PI (Production Identifier) In our case, the data provided in this segment are the date of manufacture and the product batch.
The contents mentioned above are coded, preceded by the corresponding AI (Application Identifier). The AI used are:
The code, as required by law, is accompanied by the Human Readable Interpretation (HRI), and is located at the bottom.